When you pick up a prescription, you might assume the pharmacist will give you the cheapest version of your medicine-unless your doctor says otherwise. But that’s not true everywhere. In some states, pharmacists must switch you to a generic drug. In others, they can only do it if you agree. These differences aren’t just paperwork-they affect how much you pay, whether you take your meds as prescribed, and even your health outcomes.
What’s the Real Difference Between Mandatory and Permissive Substitution?
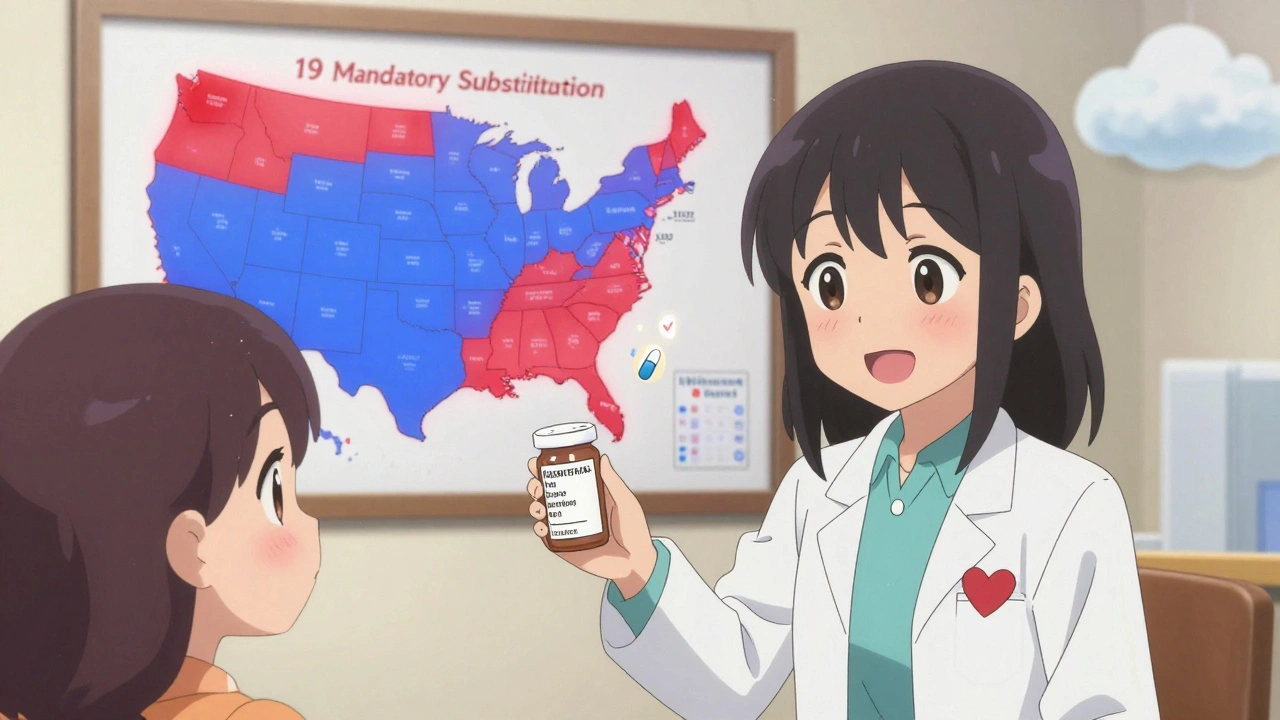
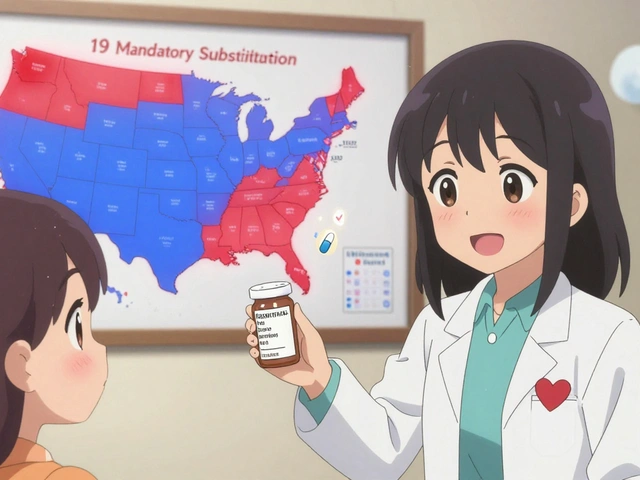
Mandatory substitution means the law forces pharmacists to give you a generic version of your drug whenever it’s available and approved by the FDA. Unless your doctor writes "Dispense as Written" or "Brand Medically Necessary," the pharmacist has no choice. This happens in 19 states as of 2020, including Alabama, Colorado, Massachusetts, and West Virginia.
Permissive substitution is the opposite. Pharmacists are allowed to swap in a generic, but they don’t have to. They can choose to give you the brand name if they think it’s better-or if they’re unsure. In these states, the decision often depends on the pharmacist’s judgment, the pharmacy’s policy, or whether you ask for the cheaper option.
The key difference? One is a rule. The other is a suggestion.
Why Do These Laws Even Exist?
The federal government doesn’t set these rules. The 1984 Hatch-Waxman Act created the system that lets generic drugs enter the market, but it left the actual substitution rules to each state. That’s why you’ll find wildly different policies across the country.
States that pushed for mandatory substitution wanted to cut costs. Generic drugs cost 80-85% less than brand names. Medicaid programs, in particular, saw huge savings when generics replaced brand drugs. A 2011 study found that in states with mandatory substitution, 48.7% of simvastatin prescriptions were filled with generics six months after the brand lost exclusivity. In permissive states? Only 30%.
But not everyone wanted that push. Some doctors and patient groups worried about safety-especially with drugs where small changes in dosage or formulation could cause big problems. These are called narrow therapeutic index (NTI) drugs. Examples include warfarin, levothyroxine, and some seizure medications. In those cases, switching drugs-even to an FDA-approved generic-can lead to side effects or treatment failure.
The Hidden Rules: Notification, Consent, and Liability
It’s not just about whether substitution is mandatory or permissive. Four other rules shape how it works in practice:
- Notification: In 31 states and Washington, D.C., pharmacists must tell you they’re switching your drug-even if the packaging says it’s generic. This might be a verbal warning, a printed note, or a sticker on the bottle.
- Consent: Seven states plus D.C. require you to give explicit permission before a generic can be given. That means the pharmacist has to ask you, "Do you want the cheaper version?" and get a yes. If you say no, they must give you the brand.
- Liability: In 24 states, pharmacists can be sued if something goes wrong after a substitution-even if the generic is FDA-approved. That makes some pharmacists hesitant to switch drugs, even when they’re legally allowed to.
- Formulary rules: Some states use a "positive formulary," listing which generics can be swapped. Others use a "negative formulary," listing drugs that can’t be substituted. Most just follow the FDA’s Orange Book, which lists approved therapeutic equivalents.
Here’s where it gets personal: in states that require consent, generic use for simvastatin dropped to just 32.1% after patent expiration. In states with no consent rule? It jumped to 98.1%. That’s not a typo. One extra step-asking you-cut generic use by two-thirds.

Biologics and Biosimilars: A Whole New Layer
It’s not just pills. Biologic drugs-like Humira, Enbrel, or insulin-are complex, expensive, and often used for autoimmune diseases and cancer. Their generic versions are called biosimilars. They’re not exact copies, but close enough to be safe and effective.
But states treat them differently. Forty-five states have stricter rules for biosimilars than for regular generics. The most common extra requirement? The doctor has to be notified before a substitution happens. Some states even require the prescriber to sign off on the switch.
Only nine states and D.C. treat biosimilars the same as regular generics. Why the caution? Because switching biologics can trigger immune reactions. And because they cost tens of thousands of dollars a year-states are afraid of making a mistake.
How This Affects You
If you’re on a chronic medication-say, high blood pressure or thyroid medicine-your state’s law can change your out-of-pocket cost dramatically. In a mandatory state, you’ll likely get the generic every time. In a permissive state, you might keep getting the brand unless you ask for the generic or your doctor blocks it.
And if you’re on a narrow therapeutic index drug? You need to know your state’s rules. Some states automatically block substitution for these drugs. Others require the pharmacist to double-check with your doctor. In a few, they can swap them without warning.
Pharmacists in states with no liability protection are more likely to avoid substitution altogether-even when it’s allowed. That means you might pay more, even if you’d prefer the cheaper option.

What You Can Do
You don’t have to wait for the law to change. Here’s what works right now:
- Ask: When you pick up your prescription, ask: "Is this the generic?" If it’s not, ask why.
- Check your prescription: Look for "Dispense as Written" or "Do Not Substitute" on the label. If you see it, your doctor blocked substitution.
- Call your pharmacy: Ask what their substitution policy is. Some pharmacies default to generics; others don’t.
- Ask your doctor: If cost is an issue, say: "Can we use the generic?" Some doctors assume you want the brand unless you say otherwise.
- Know your state: If you live in a state that requires consent, you have the right to say no. If you live in a mandatory state, you can still ask for the brand-but you’ll pay more.
Where Is This Headed?
The number of mandatory substitution states has grown-from 14 in 2014 to 19 in 2020. That trend suggests more states are prioritizing cost savings over discretion.
But as biologics become more common, regulators are adding more checks. Expect more rules around biosimilar substitution, especially around tracking which patient got which version. Some states are already requiring pharmacies to log every biosimilar switch.
Meanwhile, pharmaceutical companies are fighting back. Brand-name makers spend millions in states with permissive laws to educate doctors and patients about why their drug is "better." They push the idea that generics aren’t the same-even when science says they are.
The bottom line? The system is still messy. But if you understand the rules in your state, you can take control of your costs and your care.
Can a pharmacist substitute my generic drug without telling me?
In 31 states and Washington, D.C., pharmacists must notify you when they switch your brand-name drug to a generic-even if the packaging says it’s generic. In the other 19 states, they don’t have to tell you unless you ask. Always check your prescription label and ask if you’re unsure.
Do I have to give consent before getting a generic drug?
Only in seven states plus Washington, D.C. In those places, the pharmacist must ask you if you want the generic version. If you say no, they must give you the brand name-even if it costs more. In all other states, consent isn’t required, and substitution happens automatically unless your doctor blocks it.
Are generic drugs really the same as brand-name drugs?
For most medications, yes. The FDA requires generics to have the same active ingredient, strength, dosage form, and route of administration as the brand. They must also be bioequivalent-meaning they work the same way in your body. The only differences are in inactive ingredients like fillers or dyes, which rarely cause issues. But for narrow therapeutic index drugs like warfarin or levothyroxine, even small changes can matter. Talk to your doctor if you’re concerned.
Why do some states block substitution for certain drugs?
States often restrict substitution for drugs with a narrow therapeutic index (NTI), where even small changes in blood levels can cause side effects or treatment failure. Examples include seizure meds, blood thinners, and thyroid hormone. Some states automatically exclude these from substitution. Others require the pharmacist to check with the prescriber first. Always confirm whether your drug is on a restricted list.
Can I request a brand-name drug even if a generic is available?
Yes, in every state. But if your doctor didn’t write "Dispense as Written" or "Do Not Substitute," you may have to pay the full brand price. In mandatory substitution states, pharmacies can’t give you the brand unless you pay the difference. In permissive states, they can-but they might not stock it. Ask your pharmacist about cost and availability.
What’s the difference between a generic and a biosimilar?
Generics are exact copies of small-molecule drugs like pills or injections. Biosimilars are similar-but not identical-to complex biologic drugs made from living cells, like Humira or insulin. Because they’re more complex, biosimilars require more testing and stricter substitution rules. Most states require doctor notification or consent before switching to a biosimilar, unlike with regular generics.