Ever looked at your pharmacy receipt and wondered why a generic pill costs $4 while the brand-name version next to it is $400? It’s not a scam. It’s not a trick. It’s simple economics built into how medicines are made and approved in the U.S. And the truth is, generic drugs aren’t cheaper because they’re worse-they’re cheaper because they don’t have to pay for the same things brand-name drugs do.
They Don’t Have to Recoup Billions in Research
Every brand-name drug starts as an idea in a lab. Then comes the long, expensive climb to market: animal tests, dozens of clinical trials with thousands of patients, years of data collection, and regulatory paperwork. The average cost to bring one new brand-name drug to market? Around $2.6 billion. That’s not a typo. That’s what the Tufts Center for the Study of Drug Development found in 2014, and it hasn’t gotten cheaper since. That money has to come from somewhere. So when the drug finally gets approved, the company sets a high price. Why? To pay back investors, fund future research, and make a profit before the patent runs out. That patent lasts 20 years from the day it’s filed-sometimes longer if they tweak the formula or get extensions. During that time, no one else can legally sell the same drug. Generic manufacturers don’t have to do any of that. They don’t run the original clinical trials. They don’t spend billions proving the drug works. All they need to prove is that their version behaves the same way in your body as the brand-name drug. That’s called bioequivalence. And it’s a much shorter, cheaper path.How the FDA Makes Sure Generics Work the Same
The U.S. Food and Drug Administration (FDA) doesn’t cut corners on safety. In fact, the rules for generics are just as strict-but they’re smarter. Instead of repeating every test, the FDA lets generic makers use a shortcut called the Abbreviated New Drug Application (ANDA). This means they skip the long clinical trials and focus on one key thing: does the generic deliver the same amount of active ingredient into your bloodstream at the same speed as the brand? The FDA requires generics to match the brand within a tight range-80% to 125% of the blood concentration levels. That’s not a wide gap. It’s a narrow window designed to ensure you get the same effect. If your brand-name drug lowers your blood pressure by 15 points, your generic has to do the same. No more, no less. They also have to match the strength, dosage form, and how it’s taken-pill, capsule, injection, you name it. Even the shelf life has to be identical: generics must stay effective within 90-110% of their labeled potency until the expiration date. And they’re made in the same kind of clean rooms, under the same strict manufacturing rules (CGMP) as brand-name drugs. The FDA inspects over 12,000 manufacturing sites worldwide every year-including factories in India and China-to make sure.Why the Price Drops So Fast After the Patent Expires
Once a brand-name drug’s patent expires, the floodgates open. Dozens of companies can start making the same drug. And they all want your business. That’s when competition kicks in. In the first year after a generic enters the market, prices typically drop 80-90%. Why? Because manufacturers are fighting for market share. The more companies that make it, the lower the price goes. On average, there are 14 different makers of each generic drug. Some are big names like Teva, Mylan, and Sandoz. Others are smaller labs. But they’re all competing on price. Take Lipitor, the cholesterol drug. When it lost its patent, generic atorvastatin dropped from $500 a month to $4. Prilosec went from $300 to $6. Synthroid? Generic levothyroxine saved patients an average of $400 a month. That’s not a small discount. That’s life-changing for people on fixed incomes. The Congressional Budget Office estimates that generic competition saves the U.S. healthcare system nearly $300 billion every year. Since 2007, generics have saved Americans over $1.6 trillion. And yet, they make up only 18% of total drug spending-even though they’re used in 90.5% of all prescriptions.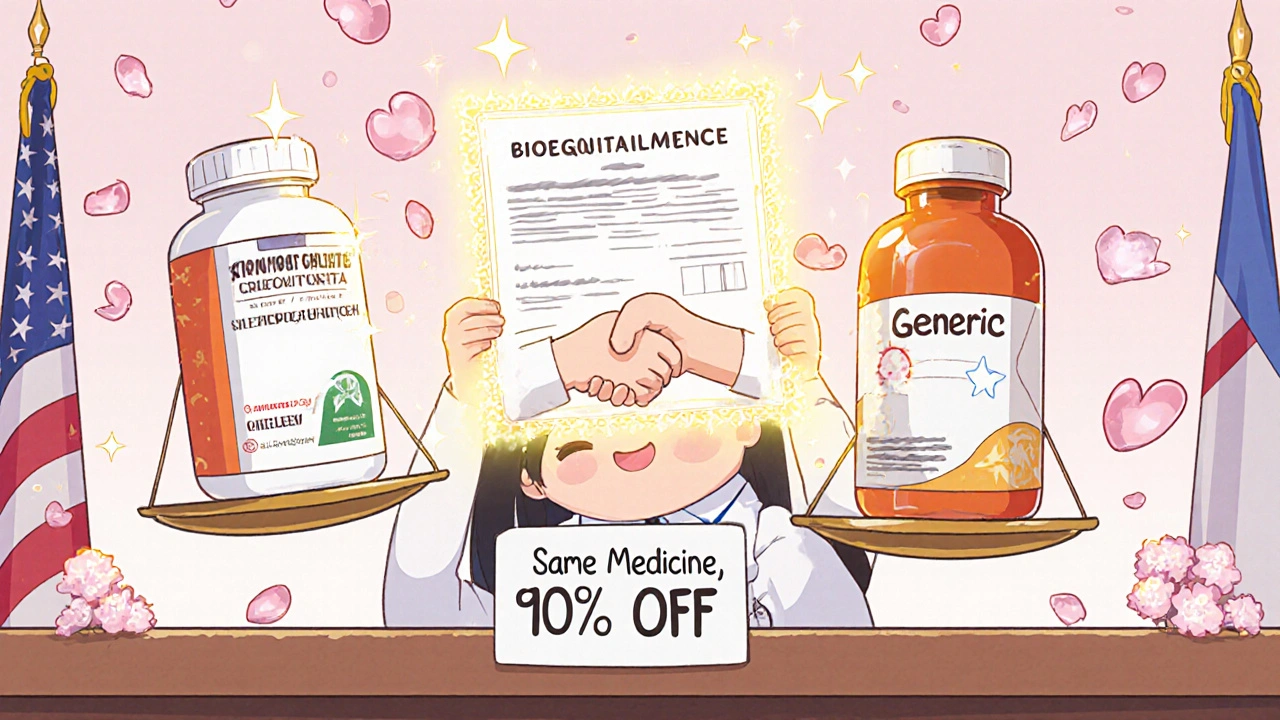
What’s Different About Generics? (Spoiler: Almost Nothing)
You might notice the pill looks different. Maybe it’s a different color, shape, or has a weird logo on it. That’s because trademark laws prevent generics from copying the brand’s appearance. But the active ingredient? Identical. The way it works in your body? Identical. The side effects? The same. The only differences are in the inactive ingredients-fillers, dyes, coatings. These don’t affect how the drug works. They’re just there to make the pill hold together or taste better. For most people, this doesn’t matter. But for a small group-like those with rare allergies or people taking drugs with a narrow therapeutic index (think warfarin or levothyroxine)-switching between different generic brands can sometimes cause minor fluctuations. That’s why doctors sometimes stick with one brand or generic version for these medications. Still, the FDA says all approved generics are safe and effective. And studies back that up. A 2021 study in the Journal of the American Pharmacists Association found that 84% of patients reported no difference in effectiveness when switching to generics. Even people with chronic conditions like epilepsy, depression, or thyroid disease generally do just as well on generics.Why Do So Many People Still Doubt Generics?
Despite all the evidence, a lot of people still think brand-name drugs are better. Tebra’s 2023 survey found that 62% of Americans trust brand-name drugs more-even though 84% admit generics are just as effective. Why the disconnect? Part of it is marketing. Brand-name companies spend billions on TV ads, doctor visits, and patient brochures. Generics? They don’t advertise. So you never hear about them. Another part is appearance. If your pill changes color or size, your brain might think something changed. One Reddit user wrote, “My doctor switched me from Concerta to generic methylphenidate and my ADHD symptoms got worse.” But in most cases, it’s not the drug-it’s the placebo effect, or maybe a different inactive ingredient causing mild stomach upset. Pharmacists are trained to explain this. In 49 states, they can automatically swap a brand for a generic unless the doctor says “do not substitute.” But many patients don’t know that. They assume the pharmacist made a mistake. That’s why the FDA launched its “Know Your Meds” campaign-to help people understand that a different-looking pill isn’t a worse one.